OVERVIEW OF ACTINIDES RECYCLE BY PYROCHEMISTRY IN NUCLEAR FUEL CYCLE
Tadashi INOUE
Central Research Institute of Electric Power Industry (CRIEPI)
2-11-1, Iwato-Kita, Komae-shi, Tokyo 201-8511, Japan
Tel: +81-3-3480-2111, Fax: +81-3-3480-7956, E-mail: inouet@criepi.denken.or.jp
The pyrochemistry provides a large potential for establishing the nuclear fuel cycle to effectively use fissile materials in fast reactors. The fuel cycle in the next generation requires lightening an environmental burden and the strong resistance for proliferation as well as an economic advantage. In order to satisfy the requirement, actinides recycle by a simply designed pyrochemical installation gives us a promising solution.
The metal electrorefining method and oxide electrorefining method are the two devices of potential. The metal fuel cycle combined with metal-fuelled fast reactor allows the on-site reprocessing proposed on early 1980¡¯s by Argonne National Laboratory (ANL)[1] and further investigated by Central Research Institute of Electric Power Industry (CRIEPI)[2]. The major devices in the pyro-process include the electrorefining and reductive extraction for collecting actinides in systems with molten chlorides and liquid metal of cadmium or bismuth. The fuel cycle[2], called as pyro-recycling system, include not only the metal fuel cycle for fast reactors but also the reduction of oxide for applying metal fuel cycle technology and the conversion of high level liquid wastes to chlorides for recovering actinides.
The electrochemical potential of actinides and lanthanides is a key fundamental property for evaluating free energy of formation of chloride in molten salts and for obtaining activity coefficients in liquid metals[3]. The distribution coefficients of actinides and lanthanides in LiCl-KCl/Cd and LiCl-KCl/Bi systems give the separation factors between actinides and lanthanides for reductive extraction.
Following the thermodynamic property, electrorefining and reductive extraction to recover actinides are examined by lots of experiments used actinide materials for technological implementation of pyrochemistry. Uranium recovery on solid cathode for electrorefining has been successfully demonstrated by an engineering scale facility with use of spent fuels in Argonne National Laboratory [4], in which the fuel conditioning program produced ca. 1.5 t of uranium ingot by treating 100 EBR-II driver assemblies and 25 DU blanket assemblies. Attempting higher collection efficiency is conducted by a kg scale of installation with use of uranium in CRIEPI [5].
The electrorefining used liquid cadmium cathode indicated to deposit plutonium with an effective collection rate by the joint study of Japan Atomic Energy Research Institute[6]. Recently, the pyroprocess facility, which can afford to treat a gram-scale of americium and hundreds gram of plutonium, was installed in the Institute of Transuranium Elements in Germany[7] This facility gives a chance to demonstrate each process of pyro-recycling system by use of an irradiated material and real high-level liquid waste.
The lithium reduction of oxides is confirmed to produce a metal of uranium, plutonium, neptunium, americium from each oxide and the mixed alloy from MOX pellet[8]. Concerning on actinide separation from high-level liquid waste, the conversion of nitrate solution to chlorides through oxides is also established through uranium tests. It is confirmed that more than 99% of TRU nuclides can be recovered by reductive extraction from simulated materials of high-level liquid waste by TRU tests[9].
Through these studies, the process flow sheets for reprocessing of metal and oxide fuels and for partitioning of TRU separation have to be established. The subjects to be emphasized for further development are classified into three categories, that is, process development (demonstration), technology for engineering development, and supplemental technology. CRIEPI also prepared a glove-box facility, in which hundreds grams of plutonium can be managed, to examine the material flow through the lithium reduction step to the distillation step of salt and cadmium with a cooperation of the Japan Nuclear Fuel Cycle Institute.
The Research Institute of Atomic Reactor (RIAR) in Russia has been involved to develop the oxide electrorefining technology for oxide fuel fabrication and treatment since the mid-1970¡¯s[10]. In this presentation, the achievement on oxide electrorefining is briefly mentioned.
REFERENCES
[1] Chang Y.I. (1989), The integral fast reactor, Nucl. Technol., 88, 129.
[2] Inoue T. and Tanaka H. (1997), Recycling of actinides produced in LWR and FBR fuel cycles by applying pyrometallurgical process, Proc. on Future Nuclear Systems (GLOBAL¡¯97), pp.646-652, Yokohama, 5-10 October.
[3] Sakamura Y., Hijikata T., Kinoshita K., Inoue T., Storvick T.S., Krueger C.L., Roy J.J., Grimmett D.L., Fusselman S.P. and Gay R.L. (1998), Measurement of standard potentials of actinides (U,Np,Pu,Am) in LiCl-KCl eutectic salt and separation of actinides from lanthanides by electrorefining, J. Alloy. Compound 271-273, 592.
[4] Benedict R.W., et al., ¡°Spent fuel treatment demonstration final report¡±, ANL-NT-106, August 1999.
[5] Koyama T., Iizuka M., Shoji Y., Fujita R., Tanaka H., Kobayashi T. and Tokiwai M. (1997), An experimental study of molten salt electrorefining of uranium using solid iron cathode and liquid cadmium cathode for development of pyrometallurgical reprocessing, J. Nucl. Sci. Technol. 34, 384.
[6] Iizuka M., Uozumi K., Inoue T., Iwai T.,
Shirai O. and Arai Y., (2000), Development of Plutonium Recovery Process by
Molten Salt Electrorefining with Liquid Cadmium Cathode, OECD Nuclear Energy
Agency, 6th International Information Exchange Meeting on Actinide and Fission
Product Partitioning and Transmutation (OECD/NEA), December, Madrid.
[7] Koyama T., Kinoshita K., Inoue T., Ougier M., Malmbeck R., Glatz J-P., and Koch L., Study on molten salt electrorefining of U-Pu-Zr alloy, Actinides 2001, 8O28, 4-9 November, Hayama.
[8] Usami T., Kato T., Kurata M., Inoue T., Sims H.E., and Jenkins A., (2001), Lithium reduction of MOX pellets, Actinides 2001, 8P26, 4-9 November, Hayama.
[9] Kinoshita K., Kurata M. and Inoue T. (2000), Estimation of materials balance in pyrometallurgical partitioning process of transuranic elements from high-level liquid waste, J. Nucl. Sci. Technol, 37, 75.
[10] Bychikov A.V., Kormilitsyn M.V., Vavilov S.K., Skiba O.V., Timofeyev G.A., and Gavrilov V.C., ¡°Pyroelectrochemical reprocessing of the spent FBR fuel¡±, Proc. on Back-end of the Fuel Cycle (Global2001), Paris, 9-13 September.
The
Current Status of R&D Efforts on Pyroprocessing in Korea
Kun Jai Lee
Korea Advanced Institute of Science and Technology (KAIST)
Daejon, Korea
Korea has a solid nuclear energy program to support the continuously growing demand on electricity. The only feasible, sustainable, and economical solution for the demand is the nuclear energy development. However, there are two issues on the nuclear energy; one is the stable supply of nuclear fuels needed for the operation of nuclear power reactors and the other is minimizing the effects of the by-product of radioactive wastes from the operation of reactors.
Recently, the pyro-process with economic and NPT resistant advantages has been spotlighted as a practical alternative for the well-known conventional wet process such as PUREX to solve these two issues. Its R&D effort was initiated by the United States in 1950's. However, it still requires a lot of R&D's to commercialize it.
The history of the R&D efforts in Korea on the pyro-process is relatively short. Even though the study on the KALIMER, Korean liquid metal reactor and the HYPER, sub-critical transmutation reactor, were initiated in early 90's, the real R&D program on their fuel supply scheme and pyro-process was started in 1997.
There are two distinct streams on the direction of the R&D efforts for the pyro-process; one applicable to the nuclear fuel cycle dedicated to the transmutation of long-lived nuclides by sub-critical reactors in associated with an accelerator and the other for reducing thermal load and enhancing the safety for the disposal of HLW.
In this paper, detailed technologies and developments efforts on Korean pyro-processing yet at the early stage of conceptualization and their impact on waste management will be discussed. Also the related and conducting organization¡¯s activities and long term plans will be briefly discussed.
Status and Prospects of the Fuel Cycle System on the Feasibility Study of Commercialized Fast Reactor Cycle Systems
Hiroshi Noda
Director, FBR Cycle System Development Office
Japan Nuclear Cycle Development Institute
Feasibility study on commercialized FR cycle systems has been carried out by a joint team established within JNC with the participation of all parties concerned in Japan since July, 1999. This research program aims to clarify various perspectives for commercializing the FR cycle by making the maximum use of its primary advantages and to enable it to become the future primary energy source in Japan. This also will suggest development strategies that correspond flexibly to diverse future social needs in the 21st century.
During the first phase of the program (JFY 1999 to 2000), the development targets were defined, then R&D achievements both in Japan and other countries and a wide range of technical alternatives for the FR cycle systems were studied incorporating innovative technologies. Based on these studies, preliminary conceptual designs for them were carried out, and their achievability of the development targets were evaluated.
The basic policy was to achieve the consistency of the fuel cycle by using the characteristics of FR core, such as burning and conversion, large allowance for impurities in fuel, and the possibility of TRU recycling. Accordingly, FR fuel cycle system could be considering in terms of ensuring safety, economic competitiveness, efficient utilization of resources, reduction in environmental burden, and enhancement of nuclear proliferation resistance. As for fuel cycle systems, the reprocessing technologies such as aqueous and non-aqueous were assessed considering low-decontaminated products and U/TRU co-extraction. Furthermore, the fuel fabrication technologies such as pelletizing, vibro-packing, and casting were examined with thee types of fuel. (oxide, metal and nitride) The several promising concepts of fuel cycle system were designed and screened up in the first phase.
In the second phase (JFY 2001 to 2005) of the program, the development targets in view of the trends in worldwide technologies were reviewed and the innovative technologies will be developed in this study using own development and solicitation of idea. And a few promising concepts of commercialized FR cycle system are going to be selected by multilateral evaluation, and these R&D tasks will be shown in ¡°Road Map¡±.
In this workshop, the status and prospects of the fuel cycle system in this study are presented.
Partitioning and Transmutation of Spent Nuclear Fuel by PEACER
I. S. Hwang, S. H. Jeong and B. G. Park.
Department of Nuclear Engineering, Seoul National University,
56-1 Shinlim-dong Gwanak-ku Seoul 151-742, Republic of Korea
Tel : +82-2-880-7215, Fax : +82-2-889-2688, E-mail : hisline@snu.ac.kr
It is envisioned that challenges of managing long-living nuclear wastes can be technically overcome by a partitioning and transmutation system, PEACER that is a lead-bismuth cooled Proliferation-resistant, Environment-friendly, Accident-tolerant, Continuable and Economical Reactor. By earlier studies, PEACER is shown to possess transmutation capability to achieve performance goals. Based on asymptotic core outlet temperature estimation using Wade, et al¡¯s method the safety of PEACER was evaluated for LOHS and LOF accidents. The PEACER displayed equivalent or better safety characteristics compared with current sodium cooled LMR designs. In order to reduce long-tern environmental risk and waste disposal cost, Tc-99 and I-129 are separated from other fission products for transmutation in blanket regions of PEACER. Depletion analysis showed that a PEACER can transmute all actinides and two fission products at two times the speed they are produced from LWR¡¯s per unit kWe-hr. Pyroprocessing is adopted for partitioning technology in a manner that is implemented by an international consortium to ensure the proliferation resistance. A very high separation factor is targeted for converting all the final waste into low-medium level waste for near-surface disposal. In addition, the cost analysis for PEACER system shows that it can be economically competitive with current LWR¡¯s.
JAERI¡¯s R&D on Pyrochemical Reprocessing of Nitride Fuel
Yasuo Arai and Kazuo Minato
Tokai Research Establishment
Japan Atomic Energy Research Institute
Tokai-mura, Naka-gun, Ibaraki-ken, 319-1195 Japan
JAERI (Japan Atomic Energy Research Institute) has proposed the transmutation of long-lived MAs (minor actinides) such as Np, Am and Cm by use of sub-critical ADS (accelerator driven system) with nitride fuel based on the double-strata fuel cycle concept. 1) Besides the construction of high-energy proton beam accelerator and the system design of transmutation plant, the technological development of separation of MAs from HLLW (high-level liquid waste), fabrication of MAs nitride fuel and reprocessing of spent fuel is the important subject investigated hereafter.
Pyrochemical reprocessing has several advantages over aqueous process in case of treating spent fuel for transmutation. They include compactness of the equipment, resistance to radiation damage, margin to criticality and nuclear proliferation resistance, which should be essential for treating highly radiotoxic spent fuel containing MAs as a primary component. In addition, JAERI¡¯s ADS will use MAs nitride fuel with N-15 enriched nitrogen in order to prevent the formation of hazardous C-14 from N-14. Pyrochemical process is expected to have the practical feasibility of recycling expensive N-15 compared with aqueous process.
The related R&D activities at JAERI include electrolysis of UN, NpN, PuN and their mixed nitride in LiCl-KCl molten salt, electrode reaction of U3+/U, Np3+/Np and Pu3+/Pu at solid (Mo, W) and liquid (Cd, Bi) electrodes, demonstration of recovery of Pu into liquid Cd cathode with high concentration, nitrogen release behavior at dissolution of metal nitrides and nitride formation in liquid cadmium besides preparation of thermodynamic datafile relating to pyrochemical process for nitride fuel.2) Typical results are summarized below and more detailed discussion will be given in the presentation.
Direct anodic dissolution in the salt and subsequent recovery of actinide metal at solid cathode were demonstrated in the electrolysis of UN, NpN and PuN, in which equilibrium potentials of the nitrides in the system were determined. Difference of the redox potential of U3+/U, Np3+/Np and Pu3+/Pu between at solid and liquid electrodes in the salt was thermodynamically analyzed by a lowering activity of actinides at liquid electrode due to the formation of intermetallic compounds with Cd or Bi. From economical viewpoint, recycling of N-15 is absolutely necessary. The results of dissolution of metal nitrides suggest that nitrogen is immediately released in the form of N2 gas during dissolution stage and 15N2 can be easily recovered from covering gas. Although nitride formation of actinides in liquid Cd is experimentally confirmed by bubbling N2 gas at 773-873K, reasonable fabrication manner of nitride granule or pellet for ADS-loading fuel has to be developed hereafter.
The results mentioned above concerned on U, Np and Pu bearing materials. On the other hand, TRU-HITEC (Module for TRU High Temperature Chemistry) is under construction at NUCEF of JAERI Tokai for the experiments on Am and Cm bearing materials. The first electrolysis test of AmN is planned from fiscal year 2003.3)
REFERENCES
[1] T. Mukaiyama, et al., Progress in Nuclear Energy, 38, 107-134 (2001).
[2] Y. Arai, et al., Proc. 6th OECD/NEA Information Exchange Mtg. on Actinide and Fission Products P&T, 11-13 December 2000, Madrid (CD-ROM) (2001).
[3] K. Minato, et al., NUCEF 2001, Oct. 31-Nov.2 2001, Tokai.
A Review of Pyrochemical Processes
Jae-Hyung Yoo
Korea Atomic Energy Research Institute
The overall characteristics of prospective pyrochemical processes for the recycling of actinides in spent fuels are reviewed in this study. Although the pyrochemical processes have not been commercialized yet, they have a lot of merits to be applied to the fuel cycle. Therefore, it would be quite a meaningful work to study their advantages, disadvantages and their technological suitabilities in the nuclear applications. In addition, some parts of pyroprocessing requiring for further development or comparison study are discussed in this study.
Development
of pyrochemical reprocessing technology of oxide fuel (MOX electro-codeposition
on Spent fuel reprocessing test)
Osamu Amano, Kunihiko Sutou
Tokyo Electric Power Company
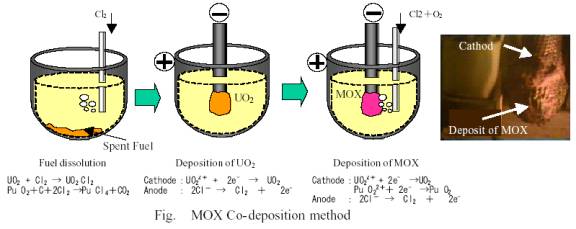
In those years it has been recognized that ¡°oxide electrolysis¡± could be one of the promising exellentary cheaper generating dry reprocessing technologies. There are two methods of Pu recovery as far as the ¡°oxide electrolytic process¡± is concerned. One is ¡°Pu Precipitation method ¡°. This is the method of Pu recovery through the precipitation. Another is ¡°MOX co-deposition method¡±. This is the method of Pu and U recovery simultaneously through the electrolysis.
Recently the test of MOX co-deposition method was conducted three times with Russian BOR-60 spent fuel at RIAR in Russia sponsored by Japanese electric utilities. In these tests, same molten salt was kept used in order to know the effect of accumulated FP.
Temperature of molten salt is around 650 C degree. Electric current potential is around 1.0 V. Thus, electrolysis is conducted by controlling both current value and partial pressure of oxygen properly.
In the first cycle of test Pu enrichment turned out to be as low as around 8%, due to the trouble with oxygen injection pipe, while 2nd and 3rd cycle showed good results of around 30% enrichment.
However, the current efficiency
(circulation current) is low by the influence of the impurities within the same
range of MOX (UO2, Pu02), the electrolysis time is long. We are investigating
with molten salt experts about the measure.
Development of Continuous Casting
Technology for the
Production of Uranium Rods
Yoon-Sang Lee, Hee-Seung Kim, Chang-Kyu Kim,
Ki-Hwan Kim, Don-Bae Lee, Seok-Jin Oh, Seong-Won Park, Young-Joon Shin
Korea Atomic Energy Research Institute
Tel: +82-42-868-8360, E-mail: yslee1@kaeri.re.kr
To reduce a
volume of spent fuel and radioactivity, an advanced spent fuel management
technology has been developed since 1997. Key procedures consist of (1)
reducing oxide fuel by lithium in a molten LiCl salt bath, (2) casting reduced
metal ingot, and (3) storing metal rod in a canister. The storage volume and
the cooling load are targeted to be reduced by a quarter and half,
respectively.
One of the
important factors of this technology is economically and safely manufacturing metal rods with a reduced
metallic fuel ingot. A directional solidification method using a vacuum furnace
and a four-zone heater was developed. However, making a long rod in this
process is not so easy, and the quartz mold byproduct of casting creates another waste disposal
problem. To make a thinner and longer rod, the continuous casting method has
been developed.
Following
experimental systems and procedures are developed for the continuous casting.
Induction power generator for melting has capacities of 75 kW and 3kHz. The
crucible is recommended to be made of graphite and coated with ZrO2 to prevent
a reaction between a crucible and melting uranium. A uranium ingot should be
melted under vacuum atmosphere with 10-5 torr. After melting an ingot, the
inert gas of Ar should be filled in a chamber. By pulling a start bar with
rollers, a solid uranium rod is continuously extracted from melt in a mold.
To identify optimum continuous casting conditions, key factors such as melting temperature, types of a mold material and starter bar, a mold temperature gradient, a withdrawal speed are examined. In parallel, computer simulation of the continuous casting process is done by the Fluent code, dedicated to analyze heat transfer in the process, to find out the optimum position for solidification.
At the optimum conditions, all melt in a crucible is completely extracted without any residues. The rod produced has an uniform diameter of 13.7 mm, and the length of 2,300 mm.
Dry pyrochemical technique for the oxide fuel decladding
Teruo Hara*1, Yuji Kosaka *2, Katsumi Imanishi*1, Kunihiko Sutou*3
*1: Mitsubishi Heavy Industries, LTD.
*2: Nuclear Development Corporation
*3: Tokyo Electric Power Company
The pyrochemical tests (heat-treated decladding and pulverization tests) were carried out using the oxide fuels which were irradiated up to 50GWd/t in the commercial PWRs, for the purpose of evaluating the applicability of the dry pyrochemical technique to the head end process. The recovery of the fuel material and the removal of the volatile fission products (FPs) were evaluated.
After a fuel pin was cut in the length of about 100mm, the single slit was given longitudinally on the fuel cladding. Then, to pulverize the fuel by oxidation, the fuel specimen was heated with 753K in the air flow. The pulverized fuel was separated from the cladding by axial vibration and recovered through the sieve.
The test results showed that most of the fuel material could be recovered and that the heating time for the fully oxidation of the irradiated fuel was longer than that of the unirradiated fuel. The experimental results showed that higher temperature of the heating was effective to shorten the oxidation time. And it showed that the heating temperature affected the particle size of the pulverized fuel.
After the pulverization and recovery by the dry pyrochemical technique, it was found that some of the volatile FPs such as Iodine was removed but majority of the Cs was retained in the fuel material during oxidation process. It was also revealed that the additional heating of the recovered fuel in the vacuumed atmosphere at 1273 K could remove the large part of the retained Cs.
Based on the experimental results, the recycle system concept for the size of 200t oxide fuel per year (LWR) and outline of the composition machine were discussed.
Hydrofluorination of metals and metal oxides
Sang Woon Kwon, Eung Ho Kim, Joon Bo shim, and Jae Hyung Yoo
Nuclear Chemical Engineering Team, Korea Atomic Energy Research Institute
The fluorination process of high-level radioactive waste is an important step to produce fluoride molten salt, which is used for the subsequent electro-separation step in the pyrochemical process. In this study, the fluorination of metals and metal oxides was investigated to prepare a fluoride molten salt. The solid reactants reacted with diluted hydrogen fluoride gas to produce fluorides in the monel tubular reactor. It was found that gas-solid reaction method was effective for the fluorination of metals and metal oxides.
Application of Uranium Fluorination Technology for Spent LWR Fuel Processing
Tetsuo
Fukasawa, Fumio Kawamura, Akira Sasahira, Yuko Kani, Youji Shibata
Nuclear Systems Division, Hitachi, Ltd.
3-1-1 Saiwai, Hitachi, Ibaraki, 317-8511
Japan
Nuclear energy produces electricity now by thermal reactors such as light water reactors (LWRs) and in the future by fast breeder reactors (FBRs) that can enhance much the utilization efficiency of uranium resource. Unfortunately realization of FBRs will be postponed, so we should consider in the near future economical and transparent LWR fuel cycle system which can recycle uranium and MOX and can also store plutonium for a while considering recent difficult conditions for MOX utilization in LWRs. Thus the authors have been developing flexible LWR fuel processing technology applicable to any kinds of LWR and FBR cycle scenarios.
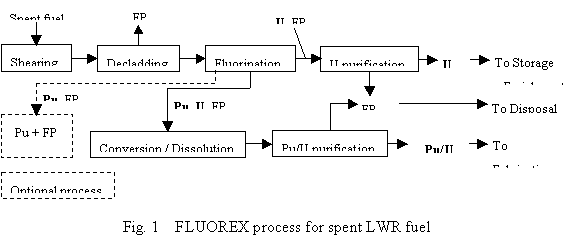
The process called FLUOREX adopts fluorination of uranium to remove main component (uranium) from spent fuel for easy treatment of residual plutonium and fission products (Fig. 1). Spent fuel from thermal reactors will be sheared and cladding material will be removed by dry oxidation/reduction method such as AIROX process. Fluorination and purification of most uranium can be easily achieved by fluoride volatility method with compact facility. About 10% residues including plutonium can be treated in well-established PUREX method, which means this facility size will be about 1/10 of the conventional PUREX facility with same capacity. Pure uranium hexafluoride product does not need conversion facility and is suitable to transfer it directly to re-enrichment (LWR again), and to store certain period for future FBRs in simple storage facilities. Pure Pu/U product can be obtained by solvent extraction method without separating Pu and U, which is suitable for conventional MOX fuel fabrication and for interim storage. Another optional process for treating fluorination residues (less than 1/20 volume of spent fuel) is to store them in a compact facility until the FBR era comes reality.
Some Novel Electrochemical Processes in Molten Chloride
Systems
Yasuhiko Ito
Department of Fundamental Energy Science, Graduate School of Energy Science,
Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan
Molten salts are, in general, chemically
and physically stable, highly electrically conductive and tough against
radiation. And in many cases, they dissolve various chemical species with
sufficiently high concentration. From an
electrochemical aspect, they have wide electrochemical window. Another outstanding feature is their
wide liquidus temperature range.
Based on such attractive features of molten salts, many molten salt
electrochemical processes have been developed and operated so far in the
world. Besides such conventional
reactions used in the above processes, various nonconventional electrochemical reactions
have been found recently in the
author's laboratory.
These new reactions might be applied to develop novel electrochemical processes in the field of material synthesis/tailoring, energy conversion, and soon. In this lecture, some selected nonconventional electrochemical reactions in molten chloride systems found recently in the author's laboratory, such as
(1) Cathodic reduction of N2 gas,
(2) Electrocheimcal implantation and displantation ,
and
(3) Discharge electrolysis,
will be described in detail.
REFERENCES
[1] Y. Ito and T.Nohira, Electrochimica Acta, Vol.45, p.2611 (2000)
[2] Y.Ito, Electrochemistry, Vol.68, p.1024 (2000)
[3] Y.Ito, Proc.6th Int.Symp.Molten Salt Chemistry and
Technology, p.150 (2001)
Status and Prospect of Low-level Radioactive Waste Vitrification
Sung-Jun Maeng,
Byueng-Chul Park, Sang-Woon Shin, Myung-Jae Song
Nuclear Environment Technology Institute, Korea Hydro and Nuclear Power Co., Ltd.
Pierre
Brunelot
SGN, France
In early 1990s Korea Hydro and Nuclear Power Co., Ltd.(KHNP) recognized the importance of volume reduction of low-level radioactive wastes from nuclear power plants as well as their safer treatment in preparation for the change in public's awareness of the environment and their attitude toward radioactive wastes. With great advantages of the vitrification technology in comparison with conventional treatments, Nuclear Environment Technology Institute jointly with SGN launched an R & D program for development of vitrification technology as an ultimate treatment method. The paper aims at presenting the status of the NETEC's challenging research program performed and the prospect for its commercial application. Results of the pilot tests performed on various simulated waste streams such as spent ion exchange resin, dry active waste, boron concentrates, etc are described. In addition, a long duration vitrification test performed in September, 2001 is summarized, which successfully demonstrated that NETEC vitrification process could be effectively applied to a commercial plant for treatment of low- and intermediate-level radioactive wastes. Based on the test results, a process data book will be issued soon to design a prototype vitrification plant which is planed to be constructed in 1994. After tests with real radioactive wastes in the prototype plant and the licensing, Korea will see a new era for safe and effective treatment of nuclear power plant wastes. Then, annual radioactive waste generation from 1000 MWe PWR could be reduced to around 35¡50 from current 150 drums.
Systematic Characteristics of Lanthanides and
Actinides under Pyrochemical and Aqueous Conditions
Hajimu YAMANA, Toshiyuki FUJII, Byung Gi PARK and Hirotake MORIYAMA
Research Reactor Institute, Kyoto University
Kumatori-cho, Sennan-gun, Osaka-fu 590-0494, Japan
Fax:81-724-51-2634, E-mail: yamana@HL.rri.kyoto-u.ac.jp
Pyrochemical processing techniques have received attentions for the future reprocessing and partitioning techniques, and assessments on their technological feasibility are now under way. Actinides and lanthanides (f-elements, hereafter) are the major elements to be treated by this technique, and thus it is desired to establish a thorough interpretation of their complicated chemical behaviors under pyrochemical conditions. In pyrochemical two-phase distribution systems consisting of molten chloride and liquid metal at high temperature, either for electrochemical separation or reductive extraction, the interaction of f-elements with the components of the melts controls their distribution equilibriums. In molten chlorides, f-element cations are considered to form chlorinated complexes which interact with the salt matrix. Their metallic states dissolved in liquid metals are considered to form chemical complex with the liquid metal by metal-metal interaction and be stabilized. These interactions are expected to show systematic variations of the thermodynamic properties of f-elements along the 4f(lanthanide)- and 5f(actinide)-series.
In aqueous separation systems, the interaction with the water molecules, so-called hydration, totally govern the behaviors of the solutes. Slight energetic changes caused by the exchange reaction of hydrated waters by complexing ligands, extractants, and adsorbing sites control the distribution equilibriums of the solutes. From a viewpoint of the comparison of pyrochemical separation and aqueous separation, assessment of the systematics of the thermodynamic properties of f-elements under pyrochemical and aqueous conditions is carried out. For this purpose, cyclic valtammetry measurements for various lanthanides were carried out. In order to enhance this systematic analysis, results of the spectrophotometric measurements of lanthanide cations in molten chloride and aqueous solution are incorporated.
Dissolution of Rare Earth Elements
in Ternary Fluoride Mixtures during Electrorefining of Simulated Spent Fuel
Sung Kyu Lee
Ajou University, Suwon, Korea
As a result of nuclear power generation, spent fuel results which is the primary form of high-level nuclear waste and highly radioactive. In Korea high level radioactive wastes are accumulated at increasing rate and they should be disposed of in suitable underground repository where the spent fuel will decay for several hundred thousand years to a level of radioactivity that is about the same as natural uranium-ore deposits. Fortunately, however, decay of long-lived radionuclides in the spent fuel can be accelerated by artificially transmuting them to short-lived or stable nuclides using nuclear transmutation technique. So transmuted, the radiotoxicity of the spent fuel can be reduced to that of natural uranium-ore deposits within several hundred years.
To effect nuclear transmutation (or accelerator transmutation) of waste system, long-lived radionuclides must be partitioned from spent fuel and then converted into suitable fuel or target for nuclear transmutation. Considering technical feasibility, proliferation resistance, safety, safeguards, economy, etc., general requirements for partitioning technology differ from country to country. Also, suitable fuel form must be developed for transmutation purpose. Metal, nitride, and molten salt forms are considered as candidate fuel forms in some countries but molten salt form was chosen because an accelerator-driven sub-critical reactor is concurrently being researched in Korea Atomic Energy Research Institute (KAERI) in the same context. In KAERI, molten fluoride salt is finally considered suitable as fuel form in view of favorable interaction between fluoride fuel and neutron as evidenced by research results of Oak Ridge National Laboratory (ORNL), U. S. A., in 1960s and 1970s. Therefore, electro-refining, other related proliferation-resistant partitioning technologies and thermodynamic database at KAERI are focused on molten fluoride salts. Accordingly, conversion of spent fuel constituents such as various metals and oxides into fluorides was carried out at KAERI to put the converted fluorides to electro-refining process where eutectic fluoride melts containing various actinides, rare earth oxides and fission products are successively electrolyzed and deposited on nickel cathode.
However, preliminary experimental results of KAERI showed that rare earth oxides only partially converted to respective fluorides and dissolution chemistry of rare earth oxides at 550¡É is proposed here as an important future research topic since oxide species in the fluoride melts affect basicity of the melt which is defined as follows:
acid + F- = base
This is based upon acid-base concept of Lux and Flood, acid + O= = base and is related to Lewis acid-base concept. For halides, the Lux and Flood concept was successfully extended.
From literature survey on cyclic voltammetric and chronopotentiometric curves, the following sequence of reactions are considered to occur in molten fluorides:
O= = O + 2e- O + O = O2, O + O= = O=2
To further understand behavior of oxide species in relevant molten fluorides caused by fortuitous addition of rare earth oxides during electro-refining of simulated spent fuel, extensive electrochemical research in the field is proposed: (1) Potentiometry;(2) Voltammetry; and (3) Spectroscopy.
On the other hand, hygroscopic nature of LiF-NaF-KF (FLINAK) mixture components incurs acid-base equilibria of water:
H2O + F- = OH- + HF OH- + F- = O= + HF
H2O is an oxobase in these equilibria and for fluoride melts such as FLINAK oxobasic properties have not been mentioned so far and their effects upon electro-refining of simulated spent fuel have to be investigated in the future research, too.
In view
of these, it is quite clear that a full understanding of the oxide species in
fluoride melts requires a careful and detailed analysis of the thermodynamics
and kinetics of the dissolution of rare earth oxides into molten
fluorides. Recently, it has been
shown by Ito et al. that a stabilized zirconia solid electrolyte can be used as
an oxide-ion concentration indicator in binary molten eutectic fluorides and
the solubility products of several kinds of oxides in this melt were
determined. For an oxide ion
concentration indicating electrode, yttria, calcia or magnesia stabilized
zirconia tubes were used. Using
suitable stabilized zirconia cell as a supplement to the previously suggested
electrochemical techniques, a comprehensive and interdisciplinary research will
be under way to understand dissolution behavior of rare earth oxides in various
molten fluorides.
Refining of Halides used for Molten Salt Technique
Tsutomu Yamamura
Tohoku University
The halides appearing in the pyro-process can be chemically refined according to generally used purification methods. Commercially available reagents usually meet the demands for the use as far as the chemical purity is concerned. However, when the chemicals are heated to be the molten salts, they might have been already contaminated with the moisture during the preceding handling. The moisture contents are usually not specified in the labels of the bottles. Chlorides are particularly hygroscopic and need to be de-moisturized before the use for some halides, particularly chlorides. Even if a sample salt is pure enough, it might react with the container the container or structural materials and introduce impurities into the melt. To avoid this contamination, the materials for the container should be appropriately chosen.
Dehydration method can be calssified into the following category.
1) Dehydration of physically adsorbed moisture by heating upto 400K.
2) Dehydration of chemically adsorbed water, or water in the crystal can be attained chemical procedures, such as:
a) Chemical treatment by gaseous HX or X2 gas in solid or molten state,¡¡where X is halogen.
b) To add NH4X to the solid or to the melt.
c) To electrolyse the water in the melts as a pretretment
d) To separate the salt by sublimation from the invlatile impurities. This method can be applied for the purification of the sample of the optical measurements.
The refined hygroscopic samples should be handled in a facilities which are separated from the atmosphere by a glove box, a simple glove back, or a sealed capsule. The material of the container of the melts should be chosen appropriately to avoid the contaminatin from the container.
Spectroscopic Analysis of Chemical Species in Molten Salts
Namjun Cho
Department of Applied Chemical Engineering, Korea University of Technology & Education,
307Gajeon-ri Byungchun-myon Chonan, 330-708 Korea
Most of the spectroscopic methods of
chemical analysis have the advantage of non-destructive measurement. And also,
they can easily be utilized for in situ analysis or on-line monitoring of
chemical species in many chemical production or processing lines. In this paper
the applicability of UV absorption and Raman spectroscopy to a molten salt
system in pyroprocessing of spent nuclear fuels was briefly reviewed.
Thermochemical consideration for pyrochemical reprocessing of nitride fuels
Hirokazu HAYASHI, Toru OGAWA and Kazuo MINATO
Japan Atomic Energy Research Institute
The Japan Atomic Energy Research Institute (JAERI) has proposed the concept of the double-strata fuel cycle, which includes transmutation cycle for burning long-lived minor actinides (MA: Np, Am and Cm) besides the current Japanese commercial fuel cycle. A combination of the nitride fuel and pyrochemical reprocessing has been chosen as a candidate for the fuel cycle of the MA burner systems.
The behavior of anodic dissolution of actinide nitrides has been studied in JAERI. For UN, it was found that both UCl3 and ternary compound UNCl are formed at the anode. UNCl is less soluble in molten salts and segregates as fine particles. For PuN and NpN, nitride chlorides do not seem to be stable enough to cause similar situation. Alternative pyrochemical process for dissolution of UN into molten salts using an oxidizing agent CdCl2 has been studied. Ternary compound UNCl is obtained at relatively low temperatures and is unstable with the oxidizing agent CdCl2 at high temperatures. UNCl is considered as an obstacle to these processes.
Thermochemical consideration with the stability diagrams is a useful method for optimizing the condition of such processes. The stability diagrams show the stable species in the system as a function of the potential, partial pressure of nitrogen, temperature and concentrations of the uranium ions. For example, one can read the decrease of the stable area for UNCl at higher temperature from the figures below. Dotted lines in the figures indicate the redox potentials of Cd(II)/Cd(0) pair with the molar ratio of CdCl2 = 0.01 in the melt. One can predict the products of the reactions between UN and CdCl2 with these conditions.
In this study, we discuss the behavior of uranium in the experimental studies of pyrochemical reprocessing (anodic dissolution and dissolution with oxidizing agents) with the stability diagrams of U-N-Cl system.
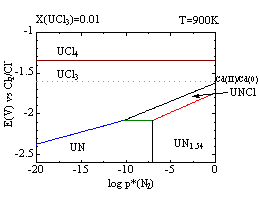
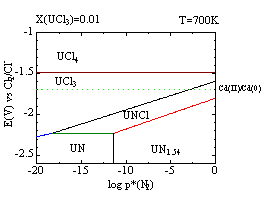
Figure Stability diagrams of U-N-Cl system in LiCl-KCl eutectic melt with the molar ratio of UCl3 =0.01 at 700K(left) and that at 900K(right), respectively. Dotted lines indicate the redox potentials of Cd(II)/Cd(0) pair with the molar ratio of CdCl2 = 0.01 in the melt.
The Safeguardability of Lithium Reduction Process
W.I.Ko, J.H.Ha, S.Y.Lee, H.D.Kim, K.S.Seo and S.W.Park
Korea Atomic Energy Research Institute
P.O. Box 105, Yusong, Taejon, Korea
The question of ¡°how to manage the spent fuel discharged from reactors¡± has been a key factor to be considered, as part of the sustainable supply of nuclear energy in the future. With the volume reduction perspective of the spent fuels to be stored and/or disposed of, a lithium reduction process has been developed in Korea. The safeguards systems, however, have not been developed, implemented, or demonstrated, so it is not known how easily international safeguards could be implemented. Generally, it is known that the dry process like the lithium process is more proliferation resistance than the current plutonium-uranium extraction process (PUREX) because the lithium process has low fission product decontamination, and plutonium is also inherently commingled with minor actinides. Those features increase apparently inherent proliferation resistance but it may make nuclear material control and accounting (MC&A) more difficult, which may be one of the safeguardability concerns of the process. In this respect, this paper addresses international/domestic safeguarding methods of the lithium reduction process and its characteristics of proliferation resistance. In order to overcome the difficulty of the MC&A, a non-destructive analysis (NDA) technique is proposed. In addition, this study adds the quantitative analysis of radiation barrier, which could be a significant accessibility barrier if the field is high enough to force a theft to shield the object during a theft. The proposed NDA concept is to measure the amount of curium in the reduction metal and associated process samples using a neutron coincidence counter and then to convert the curium mass into special nuclear material with predetermined curium ratios. For this, a well-type neutron coincidence counter with substantial shielding to protect the system from high gamma radiation is conceptually designed. About 19% of the measurement efficiency can be obtained through the simulation by MCNP (Monte Carlo N-Particle Transport) Code. From the radiation barrier analysis, it is indicated that whole-body radiation dose is about 20 rem/hr at one meter of smelt and ingot metal of 40 kgHM, which could be considered to be a significant reduction in risk of theft. It concludes tentatively that the lithium reduction process not only has inherent features of proliferation resistance but also is safeguardable.
Recovery of Uranium from Radioactive Wastes by Chloride Volatility Process
Nobuaki Sato
Institute of Multidisciplinary Research for Advanced Materials, Tohoku University
Recovery of uranium from various kinds of resources, such as ore and waste was examined by chloride volatility process. Chlorination of uranium containing fly ash was studied by using a mixture gas of Cl2 and O2 in the presence of carbon at high temperatures. Recovery of uranium from the simulated alloy waste by the chloride volatility method was also introduced.
PEACER Pyrochemical processing for Low-Level Waste Production
Byung Gi Park, Il Soon Hwang
Nuclear Materials Laboratory, Seoul National University
56-1 Shinlim-dong Gwanak-ku Seoul 151-742, Republic of Korea
A pyrochemical processing has been conceptually designed for partitioning and transmutation of spent LWR fuel by PAECER. The ultimate goal of present design is to produce low-level waste (Class C waste) for near-surface disposal. The pyrochemical processing is an efficient combination of molten salt electrolysis processes such as electrorefining, electrowinning, electropolishing, and reductive extraction. Initially, oxide fuel and zircalloy tubing from spent LWR fuel are put into molten salt and directly reduced by Li-reduction process. Most of U is electrorefined and are stored as Class C waste. Remaining U and TRU are electrowon and recycled for new PEACER fuel fabrication. Zr is also recycled for new PEACER fuel fabrication. From spent PEACER fuel, U and TRU are electrorefined and recycled. Noble metals mixed with liquid metal are separated from liquid metal with filtration and distillation. Technetium in noble metals is recovered by distillation and Iodine is recovered by off-gas handling system. Technetium and Iodine are used for making transmutation target. The used molten salt and liquid metal are decontaminated with combination of reductive extraction process and electrowinning process. Fuel assembly hardware of spent LWR and PEACER fuel are decontraminated with molten salt electropolishing. Final wastes from pyrochemical processing for PEACER are noble metals, alkaline earth metal, and lanthanides. In order to satisfy with Class C level condition, the wastes are diluted by volume. Then diluted wastes are stored at the LLW disposal site with engineered barrier.
Study on Uranium Recovery from Chemical Trap Materials By Pyro-chemical Process
Reiko FUJITA1, Hitoshi NAKAMURA1, Kiyoshi ONO1, Chiharu KAWADA1,
Ippei AMAMOTO2, Hiroshi Oobayashi2, Hiroshi UMETSU2
1Power & Industrial Systems Research & Development Center, Toshiba Corporation, Kawasaki-shi, Kanagawa 210-0862, Japan
2Ningyo-toge Environmental Engineering Center, Japan Nuclear Cycle Development Institute, Okayama 708-0698, Japan
Uranium found in the process off-gas of uranium conversion facility is removed by the chemical trap, which is charged with sodium fluoride (NaF) as fillers. As NaF has to be recharged when its adsorption efficiency becomes lowered, the amount of radioactive waste increases during long-term operation of the facility. Therefore, uranium adsorbed in NaF should be separated and reused, wherever possible. Various kinds of chemical traps are used for the removal of gaseous radionuclides, fluorine, etc in the process-off gas of uranium conversion facility or similar facilities. The spent fillers such as sodium fluoride (NaF), magnesium fluoride (CaF2), or activated alumina (Al2O3) from the chemical traps should be reused as useful materials, or converted into general industrial wastes so as to reduce the amount of radioactive waste, wherever possible.
Pyro-chemical processes for purification or recovery of actinides are well known for many decades. Electrorefining is the most common pyro-chemical process used for spent nuclear fuel. The actinides are separated from the fission products by electrotransport using a molten salt electrolyte. Recently, a pyro-chemical process has been applied to the radioactive waste treatment process which is treated the end crops of magnox spent fuel1).
A wet treatment process, fluorination volatile process, etc. can be considered as a feasible technique to be applied for this separation process. The molten salt electrolytic technique, as one of the promising uranium separation methods is discussed, and the pre-treatment condition, the electrolysis condition, the composition of molten salt and so on are clarified in this paper. The process realization of the molten salt electrolytic technique is estimated by carrying out the necessary fundamental experiments2)-5).
Molten salt technique can be applied for the removal of radionuclides from the spent fillers. The mixed salt, NaF with sodium chloride (NaCl) and the spent NaF were used in this experiment. The uranium concentration in the molten salt, pre-treatment condition, electrolytic temperature, current density, etc was used as parameters. Several fundamental experiments were carried out, and the obtained results were analyzed and evaluated. They indicate that the application of the molten salt technique is one of the potential methods for the removal of uranium from spent chemical trap materials.
REFERENCES
[1] R,Fujita, H.Namura, N.Kondo, K.Utsunomiya, S.Wade, ¡°Development of Pyro-metallurgical Process for End Crops of Magnox Spent Fuel¡±, ICONE-7, Tokyo, Japan, Apr.19-23, p186(1999)¡¡
[2] R.Fujita, H.Oobayashi, I.Amamoto, et al.,¡±Study on U Recovery Process for Chemical Trap Materials by Pyroprocess¡±, Proc.1999 Fall Meeting of the Atomic Energy Society of Japan, Kashiwazaki, Japan, Sept.10-12, 1999,725(1999)
[3] R.Fujita, I.Amamoto, et al.,¡±Study on U Recovery Process for Chemical Trap Materials by Pyroprocess¡±, Proc.2000 Fall Meeting of the Atomic Energy Society of Japan, Aomori, Japan, Sept.15-17, 2000,593(2000)
[4] R.Fujita, I.Amamoto, et al.,¡±Study on U Recovery Process for Chemical Trap Materials by Pyroprocess¡±, Proc.2001 Fall Meeting of the Atomic Energy Society of Japan, Sapporo, Japan, Sept.19-21, 2001,774(2001)
[5] I.Amamoto, T.Terai, H.Oobayashi, R.Fujita, et al.,¡±Funfamental
Study on separation and Recovery technique of Uranium from Chemical Trap
Fillers¡±, Actinides-2001, Hayama, Japan, Nov.5-9,219 (2001)
Environmental Impact of Spent Fuel Pre-Disposal Treatment Concept on
Permanent Disposal of HLW in Korea
Yongsoo
Hwang, Sunghi Kim, Chul-Hyung Kang, Jungwha Park, Seongwon Park
Korea Atomic Energy Research Institute
According to the preliminary design of a generic repository for permanent disposal of HLW, which will be inaugurated in the middle of this century, Korea needs the area of approximately four square kilometers for underground facilities to dispose of 36,000 MT from 28 nuclear power plants to be in operation or to be retired before 2015. The most preferable host rock considering geological characteristics is a crystalline rock known to have a network of fractures. Considering the ever-expanding nuclear program in Korea, the total amount of spent nuclear fuel in the future not considered in the current study, will require either big expansion of the currently proposed repository or addition of a new one. As the volume of spent fuel and the size of a repository increase, the chance to meet a major fracture zone increases. Also, a bigger size repository might cause an additional problem to secure any candidate sites during the process of site selection. Therefore, if economically feasible, it is worthwhile to develop an innovative concept to reduce the size of a potential repository. The pre-disposal treatment concept based on the lithium induced reduction process turns out to be an alternative solution to benefit the permanent disposal of HLW. The treatment concept separates nuclides with high decay heat and then stores them above ground for a certain time enough to cool down the decay heat before final disposal. The rest of HLW inclusive of the all TRU¡¯s will be transformed to a metal form and then disposed of into a repository. According to the thermal analysis for disposal of a metal form, the pre-disposal treatment concept reduces the required area for a repository at least by half. Therefore, the introduction of the pre-treatment will give more flexibility for site selection. Also, if Korea can identify a repository site with a size of four kilometers, by introducing the pre-treatment concept, Korea can dispose of all HLW not only from NPP¡¯s to be built by 2015 but also from the ones beyond that, so that Korea will be free from any political and financial burden of seeking the second repository. In addition, the metal transform alters the release mechanism of radionuclides in a waste container, which also gives a good environmental credit to the pre-treatment process by eliminating instantly high release nuclides. The technology is at the stage of early development. Therefore, it is not clear yet how much investment is required to commercialize the facilities. More attention is required to understand the financial aspect of this process throughout the stage of conceptual design of the facilities. KAERI will continuously perform a total feasibility study in the future in cooperation with domestic and international societies.